What is Li-ion Battery Construction?
lithium-ion battery, also known as Li-ion battery, is a kind of high-energy density battery. It is widely used in mobile phones, laptops, digital cameras and other mobile electronic devices due to its high voltage, high energy density, no memory effect and excellent cycles. we’ll dive deep into the design, assembly, working principles, and manufacturing processes of Li-ion batteries, shedding light on why they are the preferred choice for so many applications.
Working Principle of Li-Ion Batteries
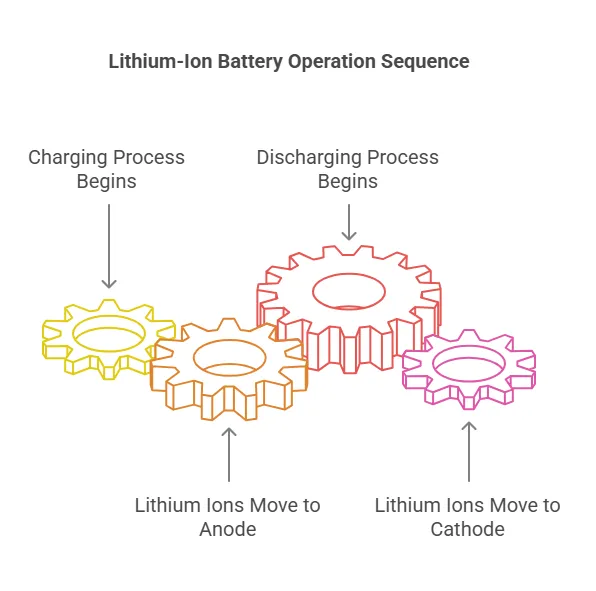
The operation of a lithium-ion battery is based on the movement of lithium ions between the cathode and anode. Here’s how it works:
The working principle of Li-ion battery is based on the principle of electrochemical reaction.
During the charging process: lithium ions are generated at the positive electrode and move to the negative electrode through the electrolyte and separator.
During discharge: the lithium ions are released from the negative electrode and move back to the positive electrode through the electrolyte and separator.
This process is repeated as long as the battery is charged and discharged.
The main components of Li-ion battery include positive electrode, negative electrode, electrolyte and separator.
The positive electrode of Li-ion battery is generally made of oxide materials containing lithium, such as lithium cobalt oxide (LiCoO2), lithium nickel oxide (LiNiO2), lithium manganese oxide (LiMnO2), etc. These materials can provide a high voltage and energy density for the battery.
The negative electrode of Li-ion battery is generally made of graphite or other carbon materials. Graphite can store more lithium ions than other materials, which can improve the capacity of the battery.
The electrolyte of Li-ion battery is generally composed of organic solvent and lithium salt (such as LiPF6, LiClO4). It plays a role of medium for ion conduction between the positive and negative electrodes.
The separator of Li-ion battery is a kind of porous film, which is sandwiched between the positive and negative electrodes to prevent short circuit between them. It also plays a role of ion conduction between the positive and negative electrodes.
During the charging and discharging process of Li-ion battery, lithium ions move through the electrolyte between the positive and negative electrodes. The movement of these ions causes the flow of electricity in the battery. The flow of electricity is directly related to the quantity of lithium ions moving in the electrolyte.
There are many different structures of Li-ion batteries, including cylindrical (eg: 4680) and flat types. The cylindrical type battery is commonly used in laptops and electric tools due to its high energy density and good structural stability. However, it has a shortcoming that it is difficult to achieve large capacity. The flat type battery has a low profile and light weight, which is convenient for mobile devices with limited space. However, its structural stability is relatively low. Therefore, different types of batteries have their own advantages and disadvantages, depending on the specific application scenarios.
The Impact of Different Packaging Forms on Batteries
The packaging forms of lithium-ion batteries vary, with common ones being cylindrical, prismatic, and pouch formats. Different packaging forms have certain impacts on the functionality and safety of batteries.
Cylindrical batteries have higher energy density and better structural stability. However, they have a larger volume and are not suitable for applications with limited space.
Prismatic batteries have lower volume and weight, making them suitable for mobile devices and tablets. However, their structural stability is relatively poorer, and they are more susceptible to external impact.
Pouch batteries have a flexible shape and structure, allowing them to adapt to various application scenarios. However, their energy density is relatively lower, and they require higher material barrier properties.
Overall, the choice of packaging form depends on the specific requirements of the application, considering factors such as energy density, structural stability, size constraints, and safety considerations.
The structure design of lithium-ion batteries needs to be adjusted according to different application scenarios. For example, in electric vehicles, battery packs need to provide high energy density and high power output, so flat or cylindrical battery structures are commonly used. In portable electronic devices, battery size and weight are crucial, so flat battery structures are typically preferred.
Typical Battery Mechanical Structure Design:
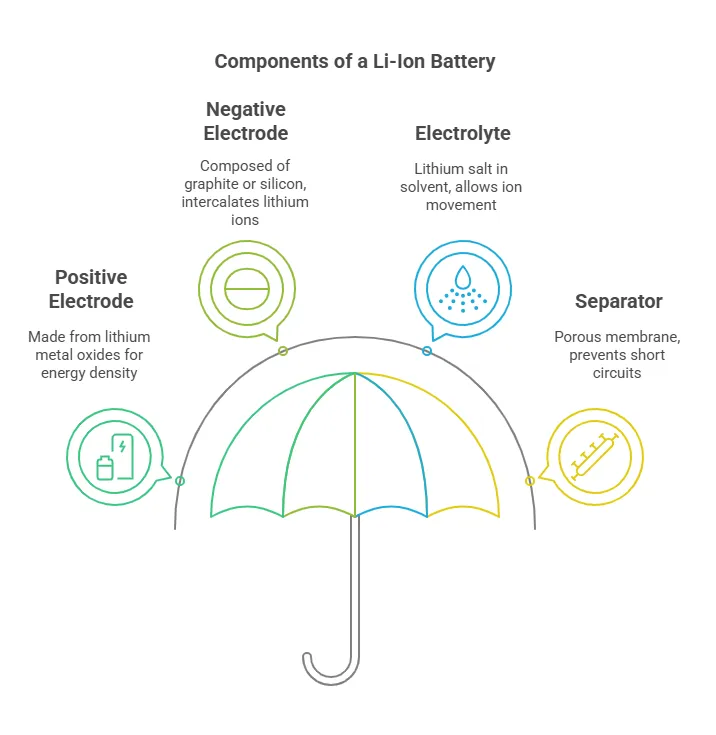
The design of a lithium-ion battery is a marvel of modern engineering. At its core, a Li-ion battery consists of four key components:
- Positive Electrode (Cathode): Typically made from lithium metal oxides like lithium cobalt oxide (LiCoO2), lithium nickel manganese cobalt oxide (NMC), or lithium iron phosphate (LiFePO4). These materials are chosen for their ability to store and release lithium ions efficiently, providing high voltage and energy density.
- Negative Electrode (Anode): Usually composed of graphite or silicon-based materials. Graphite is favored for its ability to intercalate lithium ions, while silicon offers higher capacity but faces challenges with volume expansion during charging.
- Electrolyte: A lithium salt (e.g., LiPF6) dissolved in an organic solvent. The electrolyte acts as a conductive medium, allowing lithium ions to move between the cathode and anode during charging and discharging.
- Separator: A porous polymer membrane that prevents physical contact between the cathode and anode while enabling ion transport. It plays a critical role in ensuring safety by preventing short circuits.
The manufacturing process has a significant impact on the design and performance of battery structures. Different manufacturing processes can affect factors such as the particle size, porosity, and electrode sheet thickness of electrode materials, thereby influencing the electrochemical performance and lifespan of the battery. For example, the use of laser welding technology can reduce poor contact points within the battery, improving energy density and safety. The adoption of thermal compression molding can reduce internal gaps within the battery, enhancing its capacity and cycle life.
How Li-Ion Batteries Are Assembled
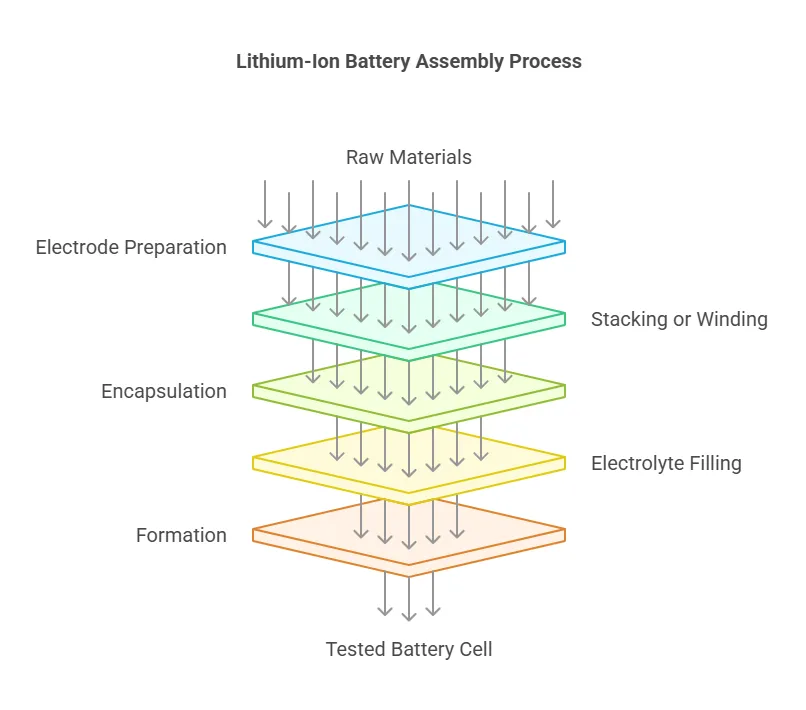
The assembly of a lithium-ion battery is a highly precise and controlled process. Here’s a step-by-step overview:
- Electrode Preparation: The cathode and anode materials are coated onto metal foils (aluminum for the cathode and copper for the anode). These coated foils are then dried and compressed to ensure uniformity.
- Stacking or Winding: Depending on the battery type, the electrodes and separator are either stacked in layers (for prismatic or pouch cells) or wound into a spiral (for cylindrical cells).
- Encapsulation: The assembled cell is placed into its casing, which can be cylindrical, prismatic, or pouch-shaped. The casing is then sealed to prevent leakage of the electrolyte.
- Electrolyte Filling: The cell is filled with the electrolyte under vacuum conditions to ensure proper wetting of the electrodes and separator.
- Formation: The battery undergoes its first charge and discharge cycle to stabilize the electrodes and form a solid electrolyte interface (SEI) layer on the anode, which is crucial for long-term performance.
- Testing and Quality Control: Each battery is rigorously tested for capacity, voltage, and safety before being approved for use.
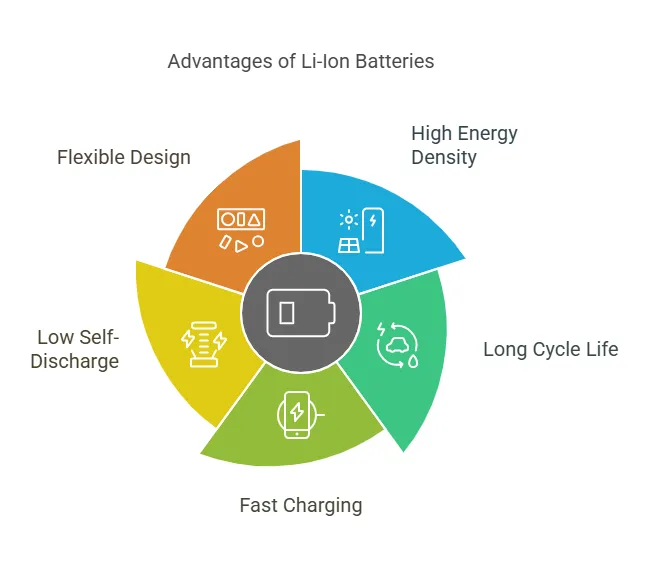
Advantages of Li-Ion Battery Construction
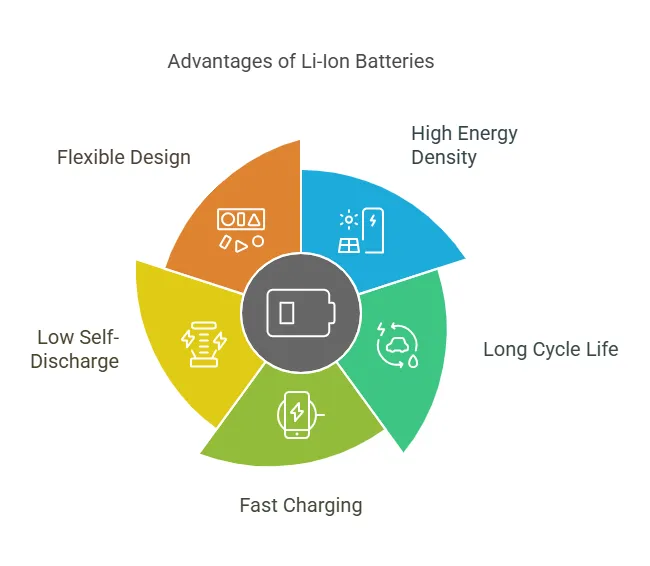
The design and construction of Li-ion batteries offer several advantages:
- High Energy Density: Li-ion batteries can store more energy per unit weight and volume compared to other rechargeable batteries, making them ideal for portable devices and electric vehicles.
- Long Cycle Life: With proper management, Li-ion batteries can endure thousands of charge-discharge cycles, ensuring long-term reliability.
- Fast Charging: Advances in electrode materials and electrolytes have enabled faster charging times without compromising safety or lifespan.
- Low Self-Discharge: Li-ion batteries lose very little charge when not in use, making them suitable for applications requiring long shelf life.
- Flexible Design: The ability to customize the shape and size of Li-ion batteries allows them to be integrated into a wide range of devices.
Li-Ion Battery Manufacturing Process Workflow
The manufacturing process of Li-ion batteries is a complex and multi-stage operation. Here’s a simplified workflow:
- Raw Material Preparation: High-purity lithium compounds, graphite, and other materials are sourced and processed.
- Electrode Production: The cathode and anode materials are mixed with binders and solvents to form a slurry, which is then coated onto metal foils.
- Cell Assembly: The electrodes, separator, and electrolyte are assembled into a cell, which is then encapsulated.
- Formation and Aging: The assembled cells undergo initial charging and discharging to activate the materials and stabilize performance.
- Testing and Sorting: Each cell is tested for capacity, voltage, and safety. Defective cells are removed, and the rest are sorted based on performance.
- Packaging: Cells are grouped into modules and packs, which include battery management systems (BMS) to monitor and control performance.
In conclusion, the design of the lithium-ion battery structure is crucial for its performance and safety. Understanding the impact of different packaging forms, application scenarios, mechanical structure design schemes, and manufacturing processes on batteries can provide a better understanding of the working principles and application range of lithium-ion batteries. It is also essential to select the appropriate battery structure and manufacturing process for different application scenarios.






