Analysis of various concepts and principles of power batteries

Follow me on:
1.Voltage (V)
Open Circuit Voltage: The voltage of a battery when it is not connected to any external circuit or load. The open circuit voltage is somewhat related to the remaining energy of the battery and is often used for battery level indication.
Operating Voltage: The potential difference between the positive and negative terminals of a battery when it is connected to a circuit and current is flowing. It is also known as the load voltage. During battery discharge, the operating voltage is lower than the open circuit voltage due to the internal resistance that needs to be overcome by the current flowing through the battery.
Discharge Cut-off Voltage: The voltage reached by the battery at the end of the discharge process when it is fully discharged. Continuing to discharge beyond this voltage can cause over-discharge, which can be detrimental to the battery’s lifespan and performance.
Charging Limit Voltage: The voltage at which the charging process transitions from a constant current to a constant voltage charging mode.
2.Battery Capacity (Ah)
Definition: Battery capacity refers to the amount of electric charge that a battery can store. It is an important parameter for battery performance and is determined by the active material in the electrodes.
Unit: The capacity is denoted by C and is expressed in ampere-hours (Ah) or milliampere-hours (mAh).
Formula: C = It, where battery capacity (Ah) equals the current (A) multiplied by the discharge time (h).
Example: For a battery with a capacity of 10 Ah, a discharge current of 5 A can last for 2 hours, while a discharge current of 10 A can last for 1 hour.
Factors affecting capacity: The actual capacity of a battery is mainly influenced by factors such as the quantity and quality of the active material and the utilization efficiency of the active material.
Rated Capacity: The capacity of a battery measured under specified conditions, as provided by the manufacturer.
Usable Capacity: The amount of electricity discharged from a fully charged battery under specified conditions.
Theoretical Capacity: The maximum capacity that a battery can theoretically release, assuming complete utilization of the active material.
3.Battery Energy (Wh)
Definition: Battery energy refers to the amount of energy stored in a battery and is expressed in watt-hours (Wh).
Formula: Energy (Wh) = Rated Voltage (V) × Operating Current (A) × Operating Time (h).
Example: The energy of a single cell with a rated voltage of 3.2V and a capacity of 15Ah is 48Wh. The energy of a battery pack with a rated voltage of 3.2V and a capacity of 100Ah is 320Wh.
Battery energy is an important parameter for measuring the ability of a battery to perform work. Capacity alone cannot determine the amount of work that can be done.
4.Energy Density (Wh/kg)
Definition: Energy density refers to the amount of energy released per unit volume or unit mass. It is commonly expressed as volumetric energy density (Wh/L) or gravimetric energy density (Wh/kg).
Example: For instance, if a lithium-ion battery weighs 325g, has a rated voltage of 3.7V, and a capacity of 10Ah, its energy density would be 113Wh/kg. The following table represents theoretical values, and in practical applications, factors such as the battery’s casing, components, and other aspects of its structure need to be considered.
Currently, lithium-ion batteries have energy densities that are three times higher than nickel-cadmium batteries and 1.5 times higher than nickel-metal hydride batteries. The energy density is determined by the material density and structure of the battery.

5.Power and Power Density
Power: Power refers to the amount of energy output by a battery per unit of time under a specific discharge regime. It is measured in watts (W) or kilowatts (kW).
Power Density: Power density, also known as specific power, is the power output per unit mass or unit volume of a battery. It is measured in watts per kilogram (W/kg) or watts per liter (W/L).
Specific power is an important indicator for evaluating whether a battery or battery pack meets the acceleration and climbing capabilities of electric vehicles.
6.Discharge Rate (A)
Definition: The discharge rate refers to the current value required to discharge a battery’s rated capacity (C) within a specified time. It is numerically equal to a multiple of the battery’s rated capacity.
Example: Taking a 10Ah battery as an example, a discharge rate of 2A corresponds to a discharge rate of 0.2C, while a discharge rate of 20A corresponds to a discharge rate of 2C.
7.Charging Modes
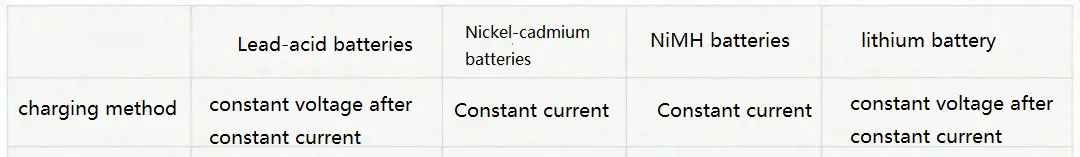
CC/CV: CC stands for constant current charging, where a fixed current is applied to the battery during charging. CV stands for constant voltage charging, where a fixed voltage is applied to the battery, and the charging current gradually decreases as the battery becomes fully charged.
Trickle Charging: Trickle charging refers to charging the battery with a current lower than 0.1C. It is commonly used when the battery is nearing full charge. If there is no strict requirement for charging time, trickle charging is recommended.
Float Charging: Float charging involves maintaining a constant voltage charging to keep the battery at a certain state of charge.
8.State of Charge (SOC) and Depth of Discharge (DOD): Representing Battery Capacity
State of Charge (SOC): The state of charge refers to the percentage of remaining capacity in a battery compared to its fully charged capacity after discharge.
Depth of Discharge (DOD): The depth of discharge is a parameter that represents the discharge level of a battery and is equal to the actual discharged capacity as a percentage of the rated capacity.
Deep Discharge: Deep discharge refers to the extent to which a battery’s capacity of 50% or more is discharged.
Example: SOC and DOD are expressed as percentages. For example, if a battery with a capacity of 10Ah is discharged to a capacity of 2Ah, it can be referred to as 80% DOD. If the same battery is charged to a capacity of 8Ah, it would be at 80% SOC. When a battery is fully charged or fully discharged, it is often referred to as 100% DOD.
9.Self-Discharge Rate (%/month)
Definition: The self-discharge rate refers to the gradual decrease in capacity of a battery during storage, expressed as a percentage of the battery’s capacity.
Causes: The self-discharge rate is primarily caused by the chemical reactions occurring between the electrodes and the electrolyte in the battery. These reactions result in the consumption of active materials and a reduction in the chemical energy converted into electrical energy, leading to a decrease in battery capacity.
Factors affecting self-discharge: Environmental temperature has a significant impact, as higher temperatures can accelerate the self-discharge of the battery.
Representation: The self-discharge rate is expressed as a percentage per month, representing the capacity decay (self-discharge rate) of the battery.
Impact: Self-discharge directly reduces the capacity of the battery and affects its storage performance. The lower the self-discharge rate, the better the storage performance of the battery.
10.Cycle Life (cycles)
Definition: In the context of rechargeable batteries, a cycle refers to one complete charge and discharge process. The capacity of a battery gradually decreases after repeated charge and discharge cycles. When the battery’s capacity drops to 80% under specific discharge conditions, the number of cycles the battery has undergone is known as its cycle life.
Factors Affecting Cycle Life: Several factors can influence the cycle life of a battery, including improper battery usage, battery materials, composition and concentration of the electrolyte, charge-discharge rate, depth of discharge (DOD%), temperature, manufacturing processes, and more.
11.Memory Effect
Definition: Memory effect in batteries refers to the reduced charging capacity of a battery that has not been fully discharged before the next charging cycle. It is the percentage of the battery’s capacity that can be charged in the subsequent charge.
Cause: The memory effect occurs when certain substances in the battery crystallize or clump together, reducing the activity of the negative electrode. For example, in nickel-cadmium batteries, the cadmium (Cd) can accumulate and form large clusters, diminishing the negative electrode’s reactivity.
Prevention: To prevent the memory effect in batteries, it is necessary to fully discharge the battery before recharging. This helps eliminate any crystallization or clumping and ensures optimal charging capacity.
Lithium-ion batteries have no memory effect.
12.Discharge Plateau
The discharge plateau refers to the portion of the discharge curve where the voltage remains relatively stable. A higher, longer, and more stable discharge plateau indicates better discharge performance of the battery.
13.Battery Pack Consistency
A battery pack is formed by connecting multiple individual battery cells in series or parallel. The overall performance and lifespan of the battery pack depend on the weakest performing cell within it, highlighting the importance of high consistency in the performance of each cell in the battery pack. In addition to the inherent performance variations among individual cells and the quality of raw materials, the manufacturing process plays a significant role. Improvements in the manufacturing process are crucial for enhancing the quality of the battery pack.
14.Formation
Formation is the process of activating the active materials in the positive and negative electrodes of a battery through specific charging and discharging methods after the battery is manufactured. This process improves the battery’s charging and discharging performance, as well as its overall performance in terms of self-discharge and storage. The true performance of a battery is realized after the formation process. Additionally, the sorting process during formation can enhance the consistency of battery packs, resulting in improved performance of the final battery pack.