Dispersion Mechanism of Lithium Battery Slurry Dispersant
Follow me on:
The electrode sheets of lithium-ion batteries are made by coating electrode slurry onto metal foils. The electrode slurry consists of the following materials dispersed in organic solvents:
Active material: Lithium-ion intercalation/deintercalation host.
Conductive additives: Facilitate electronic conduction.
Binder: Used to bond the active material and conductive additives.
For high-capacity batteries, it is necessary to reduce the proportion of conductive additives and binders while increasing the proportion of active material. However, on the other hand, it is important to ensure sufficient electronic conductivity to reduce the internal resistance of the battery and maintain electrode mechanical stability. Therefore, an appropriate amount of conductive additives and binders is required. This trade-off makes it crucial to optimize the ratio between the active material and conductive additives.
The dispersibility of active materials and conductive additives in electrode slurries is also crucial. Adequate dispersion of active materials ensures increased contact between the electrolyte and each particle’s surface, enhancing ion reactions and contributing to improved battery capacity. The degree of dispersion of conductive additives is also critical. If the electrode slurry is poorly mixed, the conductive additives cannot disperse adequately. Conversely, if the mixing is too strong, the formed electron pathways will be disrupted.
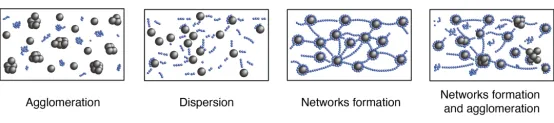
The possible internal structures of electrode slurries can be illustrated as follows:
(1) Both the conductive additives and active particles are not adequately dispersed.
(2) The conductive additives and active particles are well dispersed but exhibit weak interaction forces, resulting in their separation from each other.
(3) The conductive additives and active particles are fully dispersed, forming a structure where the conductive additives encapsulate the active particles and are linked together to create a network. This is the ideal slurry structure.
(4) When there is an excessive amount of conductive additives, partial aggregation and the formation of a partial network may occur in the slurry structure.

If the ideal slurry structure is maintained during the electrode fabrication process, it can ultimately lead to the formation of an ideal electrode structure, resulting in a well-established electron conduction pathway. This is illustrated in the following diagram.
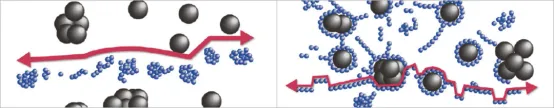
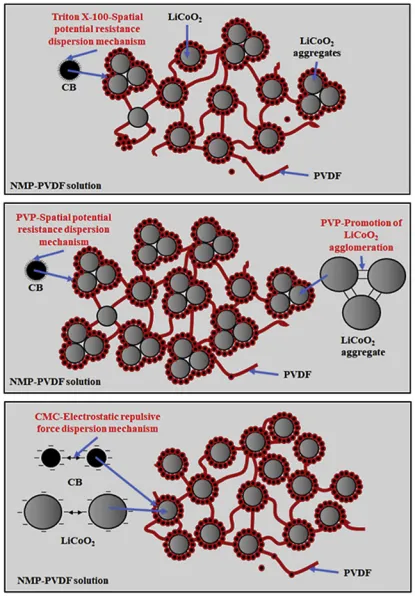
The dispersion of slurries can be achieved through mechanical methods, such as high-speed shear dispersion, or chemical methods involving dispersants. Professor Tong Zhao from Xi’an University of Technology has conducted research on the mechanisms of several common dispersants. Firstly, the optimal dosage of three typical dispersants, namely, Polyethylene Glycol Octylphenyl Ether (Triton X-100, T-100), Polyvinylpyrrolidone (PVP), and Carboxymethyl Cellulose (CMC), was determined. Subsequently, the dispersion mechanisms of dispersants T-100, PVP, and CMC in LiCoO2 lithium-ion battery slurries were elucidated.
The optimal dosages for the three typical dispersants, T-100, PVP, and CMC, are a% = 0.5%, b% = 0.5%, and c% = 1.5%, respectively. Adding the optimal amounts of dispersants to the lithium-ion battery slurry enables better dispersion of the multi-component slurry suspension and facilitates the formation of a superior internal structure compared to slurries with different mass ratios.
The dispersant T-100 has little impact on the dispersion of LiCoO2 particles. The non-ionic dispersant T-100 acts through steric hindrance dispersion mechanism on the surface of carbon black particles, effectively preventing their secondary aggregation. At the same time, the PVDF-CB bilayer tends to form around the LiCoO2 particles, facilitating the formation of a structure where the conductive additive encapsulates the LiCoO2 particles within the slurry, as shown in the upper diagram.
CMC acts on the surface of conductive additive particles and LiCoO2 particles in the slurry through the mechanism of electrostatic repulsion, effectively preventing secondary aggregation between particles. It forms a structure where the conductive additives encapsulate the LiCoO2 particles within the slurry. In summary, the ionic dispersant CMC can create a conductive pathway, a network structure of CB-encapsulated LiCoO2, and well-dispersed CB and PVDF in LiB slurries. Therefore, CMC is the optimal choice for establishing a well-structured internal architecture in lithium-ion battery slurries to enhance battery performance, as shown in the lower diagram.