The Impact of Moisture in the Lithium Battery Manufacturing Process!

Follow me on:
During the manufacturing process of lithium-ion batteries, there are three crucial factors that must be strictly controlled: dust, metallic particles, and moisture. Poor control of dust and metallic particles can directly lead to internal short circuits and fire accidents in the battery. Similarly, inadequate control of moisture can also cause significant harm to battery performance and result in serious quality accidents. Therefore, it is essential to maintain strict control over the water content of key materials such as electrodes, separators, and electrolytes throughout the manufacturing process, without any relaxation. The following will provide a detailed explanation from three aspects: the hazards of moisture in lithium batteries, the sources of moisture during the manufacturing process, and the control of moisture during the manufacturing process.
01The harm of moisture to lithium batteries
- Battery bulging and leaking
If the moisture content in a lithium-ion battery is too high, it can react with the lithium salt in the electrolyte, generating HF (hydrogen fluoride):
H2O + LiPF6 → POF3 + LiF + 2HF
Hydrogen fluoride (HF) is a highly corrosive acid that can cause significant damage to battery performance. HF can corrode the internal metal components of the battery, the battery casing, and the seals, leading to leakage and ultimately causing the battery to rupture.
HF can also disrupt the solid electrolyte interface (SEI) film inside the battery. HF can react with the main components of the SEI film:ROCO2Li + HF → ROCO2H + LiFLi2CO3 + 2HF → H2CO3 + 2LiF
Finally, the formation of LiF precipitates inside the battery leads to irreversible chemical reactions between lithium ions and the negative electrode, resulting in the consumption of active lithium ions and a reduction in the battery’s energy.
When there is a significant amount of moisture, gas generation increases, leading to an increase in internal pressure within the battery. This can result in the deformation of the battery due to the applied force, leading to risks such as battery swelling and leakage.
Instances of battery swelling or causing the device’s cover to pop open, commonly encountered in smartphones or digital electronic products in the market, are mostly caused by high moisture content and gas generation within the lithium-ion battery.
- The internal resistance of the battery increases
Internal resistance is one of the most important performance parameters of a battery. It serves as a primary indicator of the ease or difficulty of ion and electron transport within the battery, directly impacting the battery’s cycle life and operational status. A lower internal resistance results in a lower voltage drop during battery discharge, allowing for a higher energy output.
When the water content increases, it can lead to the formation of POF3 and LiF precipitates on the surface of the solid electrolyte interface (SEI) film in the battery. This disrupts the compactness and uniformity of the SEI film, gradually increasing the internal resistance of the battery. Consequently, the battery’s discharge capacity decreases continuously.
- Shortened cycle life
When the water content is too high, it damages the SEI film of the battery, leading to a gradual increase in internal resistance. As a result, the battery’s discharge capacity decreases, and the usage time per full charge also becomes shorter. The number of charge-discharge cycles that the battery can undergo before experiencing degradation naturally decreases, leading to a shorter overall battery lifespan.
02 Sources of moisture in lithium battery production process
- Moisture brought in by raw materials
- 1 Positive and negative electrode materials:The active materials in both the positive and negative electrodes of batteries consist of particles at the micro- and nano-scale, which are highly prone to absorbing moisture from the air. This is particularly true for ternary or binary positive electrode materials with a high nickel (Ni) content, as they have a relatively large surface area that facilitates the absorption of moisture and subsequent reactions. After the electrodes are coated, if they are stored in a high-humidity environment, the surface coating of the electrodes can quickly absorb moisture from the air.
- 2 Electrolyte:The solvent component in the electrolyte can undergo chemical reactions with water molecules. Additionally, the solute lithium salts in the electrolyte are also prone to absorbing moisture and undergoing chemical reactions. As a result, there is a certain amount of water content present in the electrolyte. If the electrolyte is stored for an extended period or in a high-temperature environment, the water content in the electrolyte can increase.
- 3 Separator:The separator is a porous plastic film (made of PP/PE material), and it has a significant water absorption capacity.
- Moisture added to the electrode sheet pulping:During the negative electrode slurry preparation, water is added and mixed with the raw materials before the coating process. As a result, the negative electrode sheet itself contains water. In the subsequent coating process, although there is heating and drying involved, a significant amount of moisture is still absorbed within the coating of the electrode sheet.
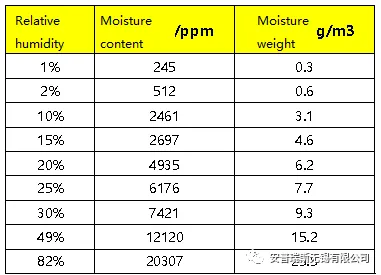
The moisture content in the air is generally measured using relative humidity. Relative humidity can vary significantly depending on different seasons and weather conditions. In spring and summer, the air tends to be more humid, with relative humidity exceeding 60%. In autumn and winter, the air is relatively dry, with lower humidity below 40%. On rainy days, the air humidity is higher, while on sunny days, the air humidity is lower. Therefore, the moisture content in the air varies depending on the different levels of relative humidity.

Water produced by the human body (human body sweat, exhaled breath, water after washing hands)

Moisture brought in by various auxiliary materials and papers (cartons, rags, reports)


03Control of moisture during lithium battery production process
1.Strictly control the environmental humidity in the production workshop
1.1 In the electrode production workshop, during slurry mixing, the relative humidity should be ≤10%.
1.2 In the electrode production workshop, during coating (head and tail), and roll pressing, the dew point humidity should be ≤-10℃ DP.
1.3 In the electrode production workshop, during slitting, the relative humidity should be ≤10%.
1.4 In the electrode stacking, winding, and assembly workshop, the dew point humidity should be ≤-35℃ DP.
1.5 During cell injection and sealing, the dew point humidity should be ≤-45℃ DP.
2. Strictly control human body and external moisture brought into the workshop
2.1 Compliance Management:
Entry into the drying workshop requires changing clothes, wearing a hat, changing shoes, and wearing a mask.
Touching the electrode sheets and cells with bare hands is strictly prohibited.
2.2 Management of Moisture Contamination:
It is strictly prohibited to bring cardboard boxes into the drying workshop.
Paper-based posters and signs inside the drying area must be laminated.
Water mopping is prohibited in the drying area.
3.Strict control of electrode sheet storage and exposure time:
3.1 Management of low humidity storage:
Electrode sheets after roll pressing and slitting must be stored in a low humidity environment within 30 minutes (≤-35℃ DP).
Electrode sheets that have been baked but not promptly processed (film winding) must be stored under vacuum (≤-95kPa).
3.2 Management of exposure time:
After baking, electrode sheets must be processed (film winding), encapsulated, injected with electrolyte, and sealed within 72 hours (workshop dew point humidity ≤-35℃).
3.3 First-in, First-out (FIFO) management:
The use of electrode sheets must follow the FIFO principle, where batches that were produced earlier are used first.
Baked electrode sheets should be used in the order of their baking.
4.Strictly control the baking process of electrode sheet and separator
4.1 Electrode sheets and separators must be baked (dried) prior to use.
4.2 If electrode sheets and separators are not baked (dried) before film winding, they must be baked (dried) before cell injection.
4.3 During the baking (drying) process of electrode sheets or cells, the parameters of the oven (temperature, time, vacuum level) must be strictly monitored.
4.4 The temperature and vacuum level of the oven must be regularly calibrated to ensure accuracy.
5. Water content testing and control
5.1 The water content of the electrode sheets, separators (or cells), and electrolyte must be tested, and only if they meet the requirements can the cell be filled.
5.2 Testing method: Sampling according to regulations and measuring using a Karl Fischer moisture tester.
5.3 Acceptance criteria for water content:
Electrode sheet water content ≤200ppm (pre-control ≤150ppm)
Separator water content ≤600ppm
Electrolyte water content ≤20ppm

In summary, in the manufacturing process of lithium batteries, controlling the environmental humidity, storage and exposure time of electrode sheets, drying and dehumidification processes for electrode sheets and separators, expiration dates of electrolytes, and testing of water content are all essential. Failure to control these factors can lead to severe defects in the performance of battery batches, with serious consequences. Therefore, whether it is management personnel, production operators, or quality inspectors, it is crucial to enhance awareness of moisture control in batteries and strictly adhere to the regulations throughout the manufacturing process to ensure that the moisture content of the batteries remains controlled and within acceptable limits.