Since its introduction to the market, lithium-ion batteries have gained widespread application due to their long lifespan, high energy density, and lack of memory effect. However, low-temperature usage of lithium-ion batteries poses challenges such as reduced capacity, severe degradation, poor cycling performance, pronounced lithium precipitation, and lithium imbalance during charging and discharging. As the application areas continue to expand, the limitations imposed by the poor low-temperature performance of lithium-ion batteries become increasingly apparent.
According to reports, the discharge capacity of lithium-ion batteries decreases to approximately 31.5% of its room temperature value at -20°C. Traditional lithium-ion batteries typically operate within the temperature range of -20°C to +55°C. However, in industries such as aerospace, military, and electric vehicles, it is necessary for batteries to function properly at -40°C. Therefore, improving the low-temperature properties of lithium-ion batteries is of great significance.
1.Factors restricting the low-temperature performance of lithium-ion batteries include:
a. In low-temperature environments, the viscosity of the electrolyte increases, and in some cases, it may even partially solidify, resulting in a decrease in the conductivity of lithium-ion batteries.
b.In low-temperature environments, the compatibility between the electrolyte, negative electrode, and separator deteriorates.
c. In low-temperature environments, lithium-ion batteries experience severe lithium precipitation on the negative electrode. The precipitated metallic lithium reacts with the electrolyte, leading to the deposition of reaction products and an increase in the thickness of the SEI layer.
d. In low-temperature environments, the diffusion system within the active materials of lithium-ion batteries decreases, leading to a significant increase in charge transfer impedance (Rct).
2.Discussion on Factors Affecting the Low-Temperature Performance of Lithium-Ion Batteries
Expert Opinion 1: The electrolyte has the greatest impact on the low-temperature performance of lithium-ion batteries. The composition and physicochemical properties of the electrolyte play a crucial role in the battery’s low-temperature performance. The challenges faced by batteries during low-temperature cycling are primarily attributed to the increase in electrolyte viscosity, which slows down ion conductivity and results in a mismatch between electron migration in the external circuit. Consequently, the battery experiences severe polarization and a significant decrease in charge-discharge capacity. Particularly during low-temperature charging, lithium-ion dendrites are prone to form on the surface of the negative electrode, leading to battery failure.
The low-temperature performance of the electrolyte is closely related to its own electrical conductivity. An electrolyte with high conductivity enables fast ion transport and allows for a higher capacity at low temperatures. The greater the dissociation of lithium salts in the electrolyte, the higher the number of migrating ions and thus the higher the conductivity. A higher conductivity leads to faster ion conduction, resulting in reduced polarization and better performance of the battery at low temperatures. Therefore, a higher electrical conductivity is a necessary condition for achieving good low-temperature performance in lithium-ion batteries.
The electrical conductivity of the electrolyte is influenced by its composition, and reducing the viscosity of the solvent is one way to improve electrolyte conductivity. Good solvent flowability at low temperatures ensures efficient ion transport. Additionally, the SEI formed on the negative electrode at low temperatures plays a crucial role in lithium ion conduction. The resistance of the SEI (RSEI) is the main impedance affecting the lithium-ion conduction in low-temperature environments.
Expert Opinion 2: The main factor limiting the low-temperature performance of lithium-ion batteries is the significant increase in Li+ diffusion impedance at low temperatures, rather than the SEI film.
3.Low-temperature characteristics of lithium-ion battery cathode materials
3.1 Low-Temperature Characteristics of Layered Cathode Materials
Layered cathode materials, which possess both the rate performance of one-dimensional lithium ion diffusion channels and the structural stability of three-dimensional channels, were among the earliest commercially used lithium-ion battery cathode materials. Representative materials include LiCoO2, Li(Co1-xNix)O2, and Li(Ni,Co,Mn)O2.
Xie Xiaohua et al. conducted a study on LiCoO2/MCMB (Mesocarbon Microbeads) to investigate its low-temperature charge and discharge characteristics.
The results showed that as the temperature decreased, the discharge plateau decreased from 3.762V (0℃) to 3.207V (-30℃). The total capacity of the battery also decreased significantly from 78.98 mA·h (0℃) to 68.55 mA·h (-30℃).
3.2 Low-Temperature Characteristics of Spinel Cathode Materials
Spinel-structured LiMn2O4 cathode materials have the advantages of low cost and non-toxicity due to the absence of Co elements.
However, the variable valence state of manganese (Mn) and the Jahn-Teller effect of Mn3+ result in structural instability and poor reversibility of this component.
Peng Zhengshun et al. pointed out that different preparation methods have a significant impact on the electrochemical performance of LiMn2O4 cathode materials, as exemplified by Rct (charge transfer resistance). LiMn2O4 synthesized by high-temperature solid-state method exhibits significantly higher Rct compared to that synthesized by sol-gel method, and this phenomenon is also reflected in the lithium ion diffusion coefficient. The main reason for this is the significant influence of different synthesis methods on the crystallinity and morphology of the resulting products.
3.3 Low-Temperature Characteristics of Phosphate-Based Cathode Materials
LiFePO4, due to its excellent volumetric stability and safety, has become a dominant cathode material for current power batteries, alongside ternary materials. The poor low-temperature performance of lithium iron phosphate (LiFePO4) is mainly attributed to its intrinsic insulating nature, low electronic conductivity, and poor lithium ion diffusion. At low temperatures, the conductivity of LiFePO4 is reduced, leading to increased internal resistance, significant polarization effects, and hindered charge-discharge processes. As a result, the low-temperature performance of LiFePO4 batteries is not ideal.
4.Low-temperature characteristics of lithium-ion battery anode materials
Compared to cathode materials, the low-temperature deterioration of lithium-ion battery anode materials is more severe, mainly due to the following three reasons:
- During high-rate charge and discharge at low temperatures, the battery experiences severe polarization, leading to the deposition of a large amount of metallic lithium on the surface of the anode. The reaction products between metallic lithium and the electrolyte generally do not exhibit conductivity.
- From a thermodynamic perspective, the electrolyte contains polar groups such as C-O and C-N, which can react with the anode material, forming a solid electrolyte interphase (SEI) film that is more susceptible to low-temperature effects.
- Carbon anodes face difficulties in lithium insertion at low temperatures, resulting in charging and discharging asymmetry.
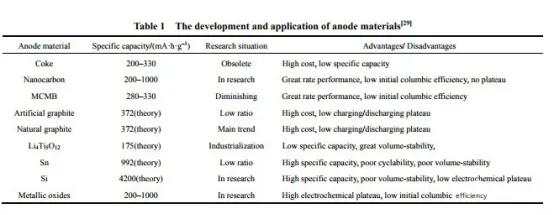
5.Research on Low-Temperature Electrolytes
Low-Temperature Characteristics of EC-Based Electrolytes Compared to Linear Carbonates, cyclic carbonates have a more compact structure and stronger intermolecular forces, resulting in higher melting points and viscosities. However, the cyclic structure of carbonates often leads to high polarity and a large dielectric constant. The high dielectric constant of EC solvent, along with its high ionic conductivity and excellent film-forming properties, effectively prevents solvent molecules from intercalating. As a result, EC plays an indispensable role in low-temperature electrolyte systems. Therefore, commonly used low-temperature electrolyte systems are mostly based on EC, combined with low-melting-point small molecule solvents.
Lithium salt is an essential component of the electrolyte. Lithium salt not only enhances the ionic conductivity of the solution but also reduces the diffusion distance of Li+ ions in the solution. Generally, the higher the Li+ concentration in the solution, the higher the ionic conductivity. However, the relationship between lithium ion concentration and lithium salt concentration in the electrolyte is not linear but parabolic. This is because the concentration of lithium ions in the solvent depends on the strength of the dissociation and complexation reactions of the lithium salt in the solvent.
6.Summary
To ensure the low-temperature performance of lithium-ion batteries, several key points should be considered:
a. Formation of a thin and dense solid electrolyte interphase (SEI) film.
b. Ensuring a large diffusion coefficient for Li+ ions within the active materials.
c. High ionic conductivity of the electrolyte at low temperatures.






