Lithium precipitation, thickening of the electrode surface passivation film, loss of reversible lithium, and degradation of the active material structure can all lead to a decline in the lifespan of lithium-ion batteries. Among them, the negative electrode is the primary factor causing capacity decay in the battery. This article summarizes the main principles of negative electrode decay during battery usage and proposes several methods to reduce capacity degradation.
The mechanisms of battery capacity degradation have been extensively studied and reported. The main factors influencing battery capacity degradation include: The main factor is the reduction in the amount of reversible lithium caused by surface side reactions on the electrode. The secondary factors include the decrease in the active material, such as metal dissolution, structural damage, and material phase transitions, as well as an increase in battery impedance. The negative electrode is related to many of the influencing factors in these degradation mechanisms.
1.Research progress on the mechanisms of negative electrode decay
Carbon materials, especially graphite, are the most widely used negative electrode materials in lithium-ion batteries. While other negative electrode materials, such as alloy materials and hard carbon materials, are also extensively studied, the research focus is mainly on controlling the morphology and improving the performance of the active materials, with less emphasis on analyzing the mechanisms of capacity decay. Therefore, the research on the mechanisms of negative electrode decay mainly focuses on graphite materials.
The decay of battery capacity includes both storage-induced decay and decay during usage. Storage-induced decay is usually associated with changes in electrochemical performance parameters such as impedance. During usage, in addition to changes in electrochemical performance, there are also mechanical stress changes and lithium precipitation phenomena.
1.1 Changes at the Negative Electrode/Electrolyte Interface
For lithium-ion batteries, changes at the electrode/electrolyte interface are recognized as one of the main causes of negative electrode decay. During the initial charging process, the electrolyte is reduced at the surface of the negative electrode, forming a stable and protective passivation film called the solid-electrolyte interphase (SEI) film. However, during the subsequent storage and usage of lithium-ion batteries, changes can occur at the negative electrode/electrolyte interface, leading to performance degradation.
1.1.1 Thickening/Composition Changes of SEI Film
The gradual decrease in power performance during battery usage is primarily associated with an increase in electrode impedance. The increase in electrode impedance is mainly caused by the thickening of the solid electrolyte interface (SEI) film and changes in its composition and structure.
Due to the fact that the SEI film does not possess the characteristics of a true solid-state electrolyte, solvented lithium ions can still migrate through the SEI film via other cations, anions, impurities, and solvent molecules present in the electrolyte. Therefore, during extended cycling or storage, the electrolyte can still undergo decomposition reactions at the surface of the negative electrode, leading to the thickening of the SEI film.
Simultaneously, as the negative electrode undergoes expansion and contraction during cycling, the surface SEI film can crack and create new interfaces. These new interfaces continue to react with solvent molecules and lithium ions, resulting in the formation of a new SEI film. As these surface reactions progress, an electrochemically inert surface layer forms on the negative electrode surface, isolating a portion of the negative electrode material from the overall electrode and causing capacity loss.
As shown in Figure 1, after prolonged cycling, the SEI film on the negative electrode surface significantly thickens.
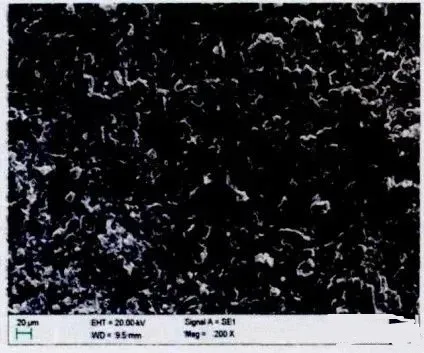
The composition of the SEI film is thermodynamically unstable and undergoes dynamic changes of dissolution and redeposition within the battery system. Under certain conditions such as high temperature, exposure to HF, or the presence of metallic impurities within the film, the dissolution and regeneration of the SEI film can be accelerated, leading to capacity loss in the battery. Particularly at high temperatures, the organic components within the SEI film can transform into more stable inorganic components (such as Li2CO3 and LiF), resulting in a decrease in the ion conductivity of the SEI film.
Research has found that different types of graphite materials exhibit varying storage performance, with synthetic graphite outperforming natural graphite at high temperatures. With increasing storage time, the lithium content in synthetic graphite remains stable, while the lithium content in natural graphite shows a linear decrease trend. Through analysis using SEM and Fourier-transform infrared spectroscopy (FTIR), it has been observed that during high-temperature storage, the surface of natural graphite exhibits a significant increase in the content of Li2CO3 and LiOCOOR with prolonged storage time. The thickening of the SEI film is primarily caused by side reactions occurring at the negative electrode surface due to electrolyte interactions. The surface structure and morphology of synthetic graphite, on the other hand, remain relatively unchanged.
1.1.2 Electrolyte Decomposition and Deposition
Electrolyte reduction includes solvent reduction, electrolyte reduction, and impurity reduction. Common impurities in the electrolyte include oxygen, water, and carbon dioxide. During the charge and discharge process of the battery, the electrolyte undergoes decomposition reactions at the surface of the negative electrode. The primary products of these reactions include lithium carbonate, fluorides, and other compounds. As the number of cycles increases, the decomposition products accumulate and cover the surface of the negative electrode, hindering the intercalation and deintercalation of lithium ions and leading to an increase in the impedance of the negative electrode.
1.1.3 lithium precipitation
Due to the close intercalation potential of graphite-based materials to lithium, if the deposition of metallic lithium or the growth of lithium dendrites occurs during the charging process, subsequent reactions between lithium and the electrolyte will accelerate the degradation of battery performance. Extensive lithium precipitation can cause internal short circuits and thermal runaway. Factors that increase the risk of lithium precipitation include low-temperature charging, a lower excess of negative electrode relative to the positive electrode, electrode size mismatch (positive electrode overlapping the edge of the negative electrode), and potential effects (differences in local polarization, electrode thickness, and porosity).
The level of disorder within the graphite material and the non-uniformity of current distribution can both affect lithium precipitation on the surface of the negative electrode. During the third and fourth stages of graphite embedded lithium, the material’s disorder leads to uneven distribution of charges within the electrode, resulting in the formation of dendritic deposits. The growth of deposits between the separator and the negative electrode is closely related to temperature and current density. As temperature increases and charging rates accelerate, the reaction rate speeds up, and metallic lithium deposits on the negative electrode. The occurrence of lithium precipitation can be determined by observing voltage plateaus in the battery’s discharge curve and a decrease in coulombic efficiency.
Currently, research primarily focuses on improving the performance of the negative electrode through various approaches such as improving the negative electrode system and optimizing the electrolyte system by incorporating additives to inhibit lithium precipitation. Coating Sn and carbon on the surface of graphite improves the electrochemical cycling performance of the negative electrode. The presence of Sn on the graphite surface reduces the internal resistance of the SEI film and electrode polarization at low temperatures. Additionally, performance enhancement can be achieved by modifying the surface of the negative electrode material. Oxidizing graphite in air increases the surface area and the number of active edge sites, leading to increased porosity and decreased particle size, thereby reducing the occurrence of lithium precipitation resulting from uneven charge distribution.
The addition of AsF6 improves the stability of the negative electrode at high temperatures, inhibits the formation of metallic lithium, and prevents the decomposition of LiPF6. Furthermore, the mechanical roll pressing during the negative electrode sheet preparation stage can reduce pore size, decrease the non-uniformity of charge distribution, and enhance the reversible capacity of the battery.
1.2 Changes in Negative Electrode Active Materials
During the gradual deterioration of battery performance, the ordered structure of graphite is gradually disrupted. In lithium-ion batteries, cycling at high rates creates a concentration gradient of lithium ions, leading to the generation of a mechanical stress field within the material. As a result, the crystal lattice of the negative electrode undergoes changes, and the initial layered structure of the negative electrode gradually becomes disordered. However, these structural changes are not the main cause of battery performance degradation. Degradation can manifest in the form of lithium precipitation or changes in the SEI film. Nevertheless, during this process, the particle size and lattice parameters of the negative electrode do not undergo significant changes.
The reversible capacity of graphite particles is influenced by their orientation and morphology. For example, due to the presence of new interfaces between disordered particles, lithium-ion/electrolyte reactions can occur, making it more difficult for lithium ions to intercalate. As a result, disordered graphite particles have lower reversible capacity. Compared to spherical particles, flake graphite exhibits higher specific capacity at high rates. Although the negative electrode structure does not change during degradation, the proportion of rhombohedral/hexagonal structures can vary. An increase in hexagonal structures reduces the Faradaic efficiency of lithium ion intercalation in the first and third stages, thereby lowering the reversible capacity of the negative electrode. Therefore, improving the proportion of rhombohedral/hexagonal structures can enhance the reversible capacity.
1.3 Changes in Negative Electrode Structure
The particle size of graphite material has a significant impact on the performance of the negative electrode. Smaller particle sizes can shorten the diffusion path between graphite particles, which is advantageous for high-rate charging and discharging. However, small-sized materials have a larger specific surface area, which can lead to increased lithium consumption at high temperatures, resulting in an increase in irreversible capacity of the negative electrode. Therefore, the thermal stability of graphite electrodes is mainly related to the particle size of the graphite material.
The porosity of graphite electrode sheets is related to the reversible capacity of the negative electrode. An increase in porosity leads to a larger contact area between graphite and the electrolyte, resulting in increased interface reactions and a decrease in reversible capacity. During the long-term cycling of the battery, the compaction density of the graphite electrode affects the performance degradation. High compaction density can reduce the electrode’s porosity, decrease the contact area between graphite and the electrolyte, and thereby improve the reversible capacity. Additionally, at temperatures above 120°C, the high-density negative electrode material generates more heat due to the thermal decomposition of the SEI film.






